Blog
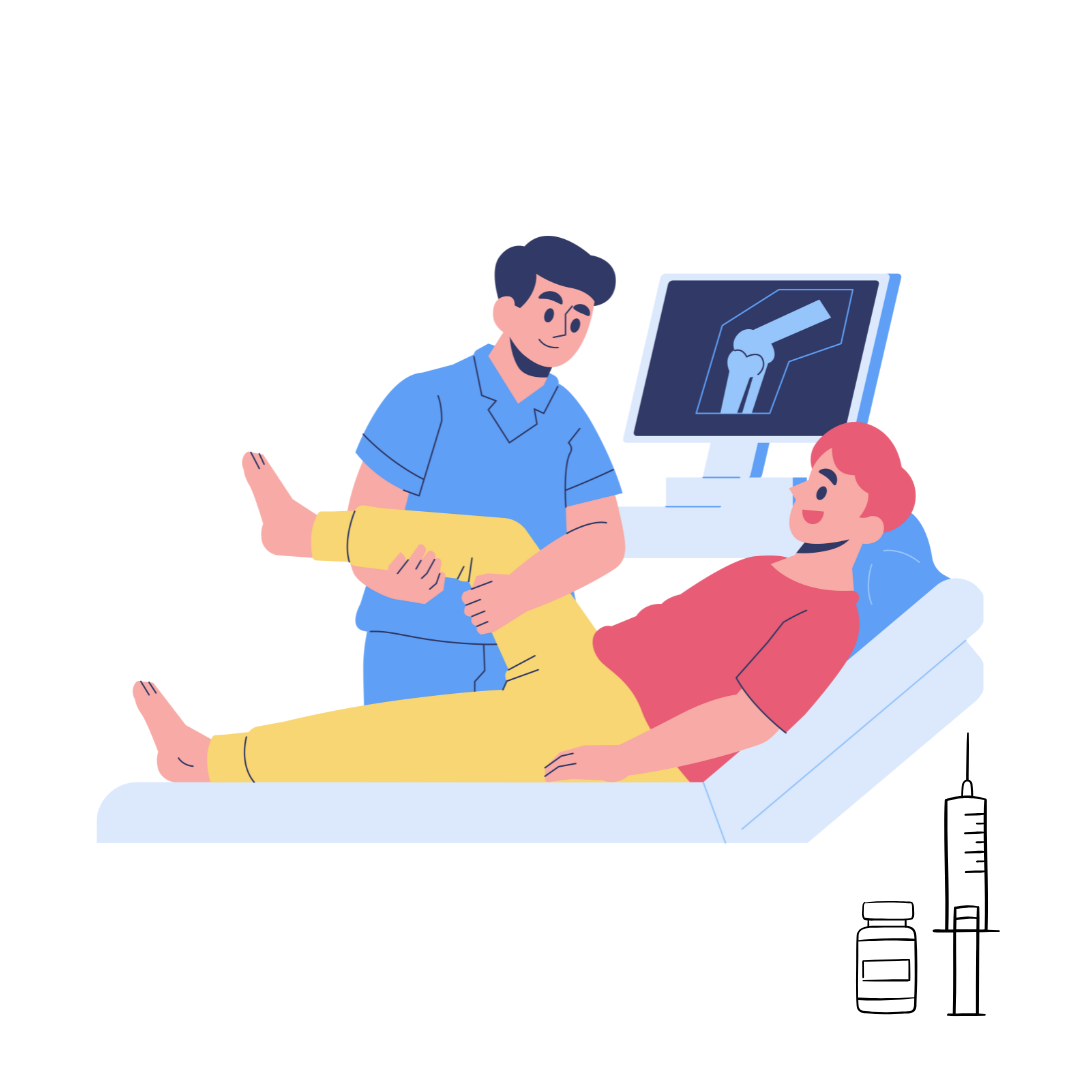
How Botox Is Offering New Hope for Pain Relief in Orthopedic Conditions
Botox, often known for smoothing wrinkles, is now being explored as a treatment for pain in certain orthopedic conditions. Recent research shows that Botox injections may help with chronic pain in conditions like tennis elbow, where traditional treatments often fall short. While there’s strong evidence that Botox can be effective for this type of pain, its use in other orthopedic issues, like knee pain after surgery or arthritis, isn’t as clear yet. More research is needed, but for some people, Botox could offer a new way to manage pain and improve mobility when other methods aren’t working
Read More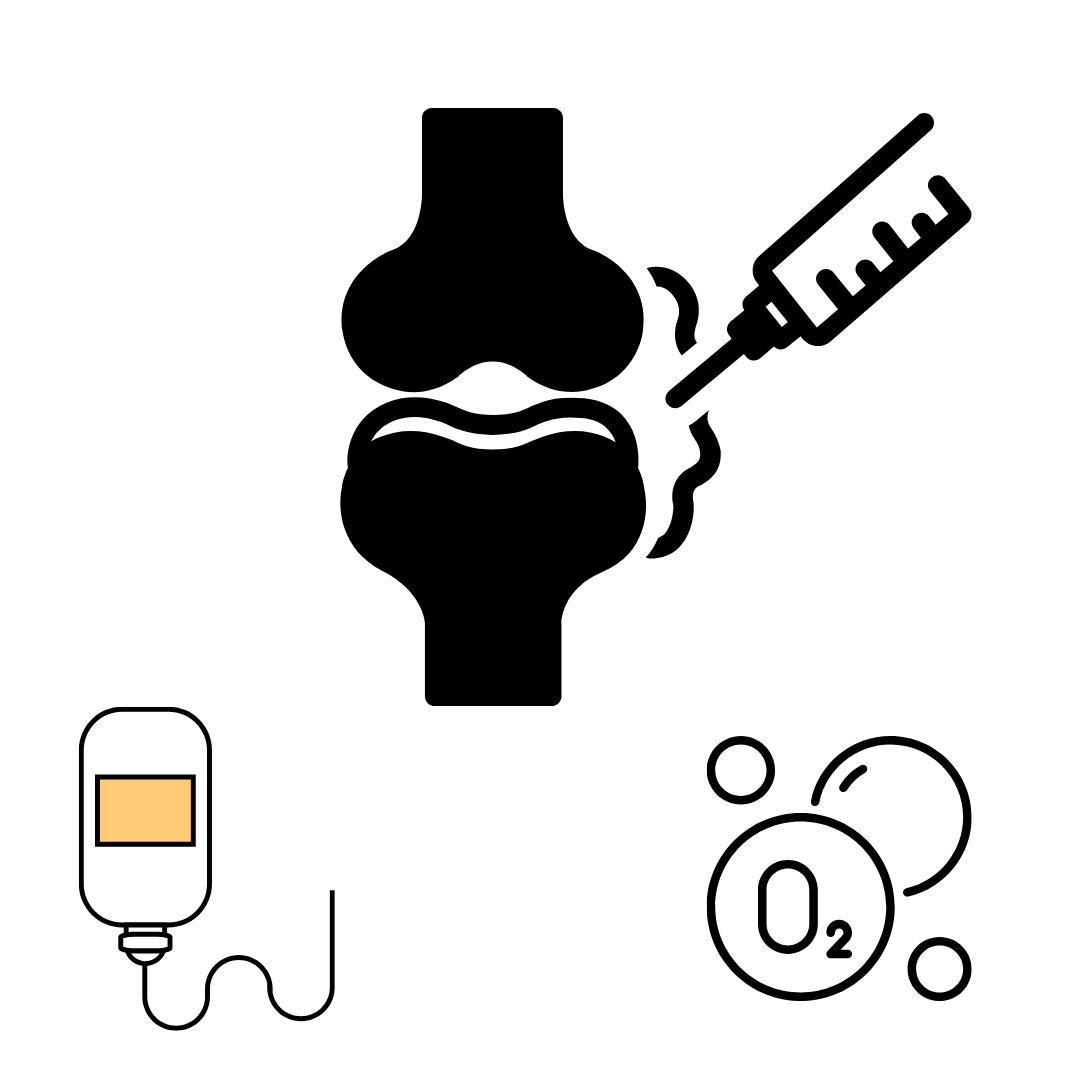
Dextrose vs. Prolozone: Comparing Two Effective Injection Therapies for Knee Osteoarthritis Relief
A recent study explored two promising regenerative treatments for knee osteoarthritis (KOA): prolotherapy using hypertonic dextrose and prolozone therapy (injections of ozone). Both treatments aim to reduce pain and improve joint function for people suffering from mild to moderate KOA.In this trial, 80 patients were divided into two groups. One group received dextrose injections, while the other received prolozone. Each patient received three injections over 10 days. After three months, researchers measured improvements in pain and function using two well-known assessment tools: the Visual Analogue Scale (VAS) and the Western Ontario and McMaster Universities Arthritis Index (WOMAC).The results showed that both groups experienced significant pain relief and better knee function. However, there was no notable difference between the two treatments — both were equally effective. This study suggests that whether patients choose dextrose or ozone injections, they can expect similar improvements in their KOA symptoms. It also highlights the need for further research to confirm long-term benefits.
Read More
Radiofrequency Ablation: A Promising Treatment for Chronic Abdominal and Thoracic Pain
This study looked at how well a treatment called radiofrequency ablation (RFA) works for people suffering from chronic pain in the abdomen and chest. Chronic pain in these areas can be difficult to manage, especially when standard treatments, like medications or physical therapy, don’t provide relief. RFA is a procedure where heat is used to target and destroy specific nerves that send pain signals to the brain. The researchers reviewed many studies from 1992 to 2022 to see if RFA helps reduce pain in people with chronic thoracic and chronic abdominal disease states. Out of 575 studies they found, 32 were included in the review. The results showed that RFA provided significant pain relief for most patients, with an average success rate of 84%. The procedure was especially effective for people with certain types of cancer, spinal tumors, and chronic post-surgical pain. However, the researchers noted that more high-quality studies (such as randomized controlled trials) are needed to confirm how well RFA works in the long term, as most current studies are observational. Despite this, RFA appears to be a promising option for people who don’t find relief from other pain management methods.
Read More
The Impact of Exercise on Depression and Suicide Prevention: A 2023 Meta-Analysis
A 2023 meta-analysis found that moderate- to vigorous-intensity aerobic or resistance exercise, excluding mind-body activities like yoga, effectively reduced depressive symptoms with a comparable efficacy to psychotherapy and medication. The analysis also revealed that exercise may decrease suicide attempts in people with mental or physical illnesses. However, addressing psychological barriers such as low motivation and fatigue is crucial for successful exercise implementation, particularly as previous studies involved willing participants with professional support. Clinicians should prescribe specific exercise regimens, ideally supervised, and use behavioral change techniques to increase adherence. Exercise interventions are generally safe, though patients should be screened for pre-existing conditions to ensure suitability
Read More
How Phototherapy is Revolutionizing Pain Relief and Recovery
Phototherapy, or light therapy, is an exciting treatment option that’s being used in new ways to help with a variety of health conditions. While it has long been used to treat skin issues and newborn jaundice, recent studies show that different types of light may also help with pain relief, vision problems, and even conditions like migraines and fibromyalgia. Green light, for example, has been found to reduce pain from chronic conditions, while red light is being used to speed up muscle recovery for athletes. Though research is still developing, light therapy is showing promise as a natural, non-invasive way to treat health issues that are hard to manage with traditional medications.
Read More